As the H5N1 avian influenza virus continues its rampage through U.S. dairy cow herds, it has also infected human farm workers. A different strain has also infected workers on poultry farms, most recently in Washington State. On Wednesday the U.S. Department of Agriculture announced that the virus had been detected in a pig for the first time at a farm in Oregon. Now, as the usual seasonal flu season approaches, some health experts wonder if it might give bird flu a dangerous boost.
There have been at least 39 human H5N1 cases in the U.S. this year. Fifteen were in California, 10 were in Colorado, nine were in Washington State, two were in Michigan, one was in Texas, and one was in Missouri. (A second person in Missouri was likely also infected, but their blood test results didn’t meet the official definition of a “case.” And officials say they have ruled out person-to-person spread there.) Known cases have mostly been mild, characterized by minor eye infections and respiratory symptoms.
Apart from the Missouri case, all of these people had confirmed contact with infected farm animals. All nine of the Washington State cases and nine of those in Colorado involved workers on farms that culled infected chickens. The remainder of cases were dairy farm workers. A total of 395 cow herds have tested positive for H5N1 across 14 states.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The steady uptick in cases—in both farm animals and humans—has some experts worried about the risk of a wider outbreak of this potentially pandemic-causing virus. Influenza viruses have several features that make them well suited for this: for one, they constantly mutate in a process known as genetic drift, which is why you need a new flu shot every year. If there are enough mutations of the right kind, the virus undergoes a quantum leap known as genetic shift, which can make it capable of unleashing a pandemic.
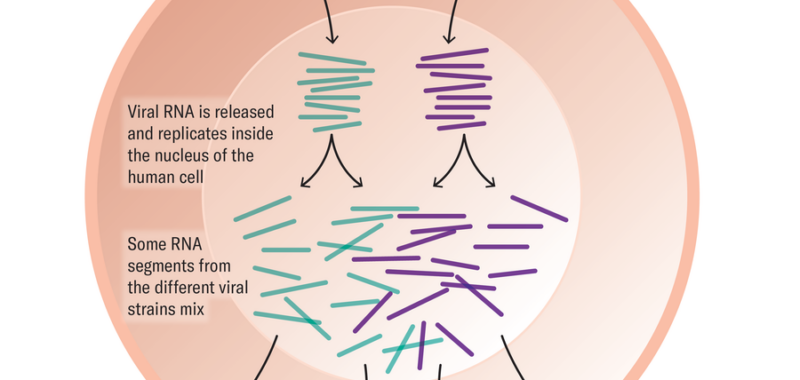
Another tool in an influenza virus’s kit is something known as reassortment. A flu virus’s genetic material is made up of eight RNA segments. When multiple viruses infect the same cell and replicate, they can swap these segments, producing one of 256 possible combinations. This reassortment can create a virus that contains features of both parent viruses, which could make it more transmissible and virulent. The process is thought to have produced the 2009 H1N1 swine flu from a mix of U.S. and European strains of pig flu virus, launching a (thankfully mild) pandemic.
Could such reassortment occur if a person were infected with both the H5N1 bird flu virus and seasonal influenza at the same time, leading to an H5N1 version that would be more transmissible in people? That’s certainly possible, experts say. But reassortment alone cannot create a virus capable of launching a human pandemic, says Richard Webby, an infectious disease researcher at St. Jude Children’s Research Hospital in Memphis, Tenn. The virus would also need to accrue certain specific mutations.
“To get from where we are now to a pandemic virus, reassortment alone—in my mind, at least—is not going to get us there,” says Webby, who directs the World Health Organization Collaborating Center for Studies on the Ecology of Influenza in Animals and Birds. “It’s going to take reassortment, followed by some critical mutations in [one specific] gene.”So far none of the key mutations necessary for the virus to spread efficiently among humans has been detected in any of the genetically sequenced human cases.
If H5N1 does develop those mutations, reassortment could help move the virus from an infected human’s eye (the site of most known farm worker infections) to their respiratory tract, Webby says. If it occurred at all, such mixing would most likely happen in a human host, he says. Although cows can get infected with human flu viruses, it’s less likely that those viruses would replicate in the cows’ udders, which is where H5N1 seems to replicate best.

Historically, pigs have been viewed as the ideal mixing vessels for pandemic pathogens because they are susceptible to both human and avian influenzas. Spillovers of human seasonal viruses into pigs happen fairly regularly, says Amy Baker, a research veterinary medical officer at the USDA. Baker and her colleagues have shown that the 2.3.4.4b strain of H5N1 that is currently circulating in wild birds and dairy cows can replicate in pigs.
The pig that tested positive for H5N1 in Oregon was housed in a backyard farm with poultry and other animals. It’s not yet clear if the pig transmitted the virus to any other animals, but health authorities are investigating. All five pigs on the farm have been euthanized. Because the farm is a noncommercial operation, there is no concern about the nation’s pork supply, USDA officials said in a recent statement.
“This does seem to be a pretty limited episode on a backyard farm, so I think in itself, it doesn’t pose any particular danger, assuming there wasn’t any movement of animals to other farms,” Webby says. But if this represents an actual infection of pigs and not just a positive nasal swab, he says, “it does suggest that they are naturally susceptible to the virus.”
If H5N1 were to start infecting pigs on commercial hog farms, that would heighten the chances of reassortment with seasonal influenza. “We know reassortment happens a lot in pigs—there are viruses in pigs that are very closely related to those humans. So, it would absolutely, absolutely increase the risk.”
There are still many unanswered questions about how the H5N1 virus got into cattle in the first place and began spreading, Baker says. She agrees with Webby that there is little risk of the virus reassorting with human seasonal flu viruses in cows because there is no evidence of the latter pathogens infecting the animals. But if a pig or person were to be coinfected with both viruses, she says, there is “always a chance” it could create a more dangerous hybrid virus.
This risk is a reason the Centers for Disease Control and Prevention has urged farm workers to get their seasonal flu shots. The U.S. has a stockpile of H5N1 vaccines, but it has not yet distributed any. There is some concern that low trust in vaccines could affect uptake. It remains unclear what various officials’ threshold for deploying H5N1 vaccines among farm workers and other susceptible individuals might be, although evidence of sustained human-to-human transmission would likely be a strong factor.
“It’s not a bright-line rule,” said the CDC’s principal deputy director Nirav Shah to Scientific American at a press briefing last week. “It’s really a variety of factors that we think about as we evaluate the pros and cons of vaccination.” These include the emergence of person-to-person spread and increasing virulence or severity of illness—and none of these factors has yet been seen, he added. In the meantime, people infected with H5N1 and their close contacts are being treated with the medication oseltamivir (Tamiflu).
Some scientists have called for vaccinating cattle against H5N1, and the USDA’s Center for Veterinary Biologics has approved a couple of vaccine field safety trials. “I think there’s an opportunity for using H5 vaccines in cattle because it’s the only subtype that have knowledge of infecting cattle at this point,” Baker says. “And if we could reduce the amount of virus that’s being shed through the milk, I think that will be a benefit to both the milk production side of it, as well as protecting the farm workers and the public.”
Right now the chances of a farm worker getting H5N1 at the same time as seasonal flu are low,Webby says. But as flu season ramps up this winter, that risk could increase. Hundreds of humans have been infected with avian flu in the past quarter-century, and it hasn’t yet started transmitting widely among us. That fact, Webby says, suggests “the hurdles are high that this virus has to overcome to become a human virus. But anything that gives it more opportunity to do so is obviously a concern—whether that’s just more human infections from farm animals or that potential of reassorting with a human seasonal virus. All of those things would increase the risk.”